WHO Raises Ethical Concerns Over US-Funded Vaccine Trial in Guinea-Bissau
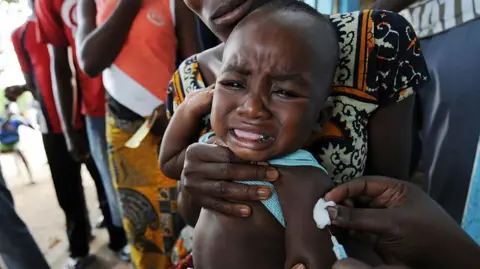
The World Health Organization (WHO) has criticized a now-halted plan to conduct a hepatitis B vaccine trial involving thousands of newborns in Guinea-Bissau, labeling it as unethical. The trial, which was funded by the US and cost $1.6 million, aimed to assess the effects of administering the vaccine at birth compared to delaying the shot until six weeks of age.
The WHO expressed significant concerns about the study's scientific justification, ethical safeguards, and its alignment with established standards for research involving humans. Highlighting the effectiveness of the birth-dose vaccine—proven to reduce mother-to-baby transmission of hepatitis B by 70-95%—the organization called for immediate precautions.
Under the helm of Robert F Kennedy Jr, a vocal vaccine skeptic, the US health department initiated the trial to explore broader health effects linked to the vaccine. However, the WHO stressed that giving a life-saving intervention to some newborns while withholding it from others was potentially harmful.
The proposal faced immediate backlash from public health advocates and the Guinea-Bissau government, leading to its suspension amid fears of unethical experimentation. There were plans to recruit approximately 14,000 babies for the study, which critics—including former health minister Magda Robalo—deemed unacceptable, emphasizing that the citizens of Guinea-Bissau should not be treated as test subjects.
Currently, the hepatitis B vaccine is administered at six weeks to infants in Guinea-Bissau. However, there are efforts to introduce the birth dose universally by 2028. The WHO insists that this birth dose is a critical public health measure, particularly given that more than 12% of adults in the country are chronically infected with the virus.
In light of recent developments, the WHO has reiterated its recommendations for immediate vaccination of all newborns against hepatitis B within the first 24 hours of life, as infections at birth can lead to chronic conditions, cirrhosis, and cancer later in life.